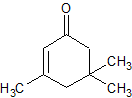

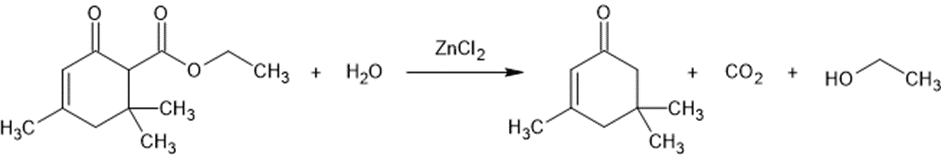
3,5,5-Trimethyl-2-cyclohexen-1-one-6-carboxylic acid ethyl ester (12.0 g), H2O (500 ml), and ZnCl2 (3.0 g) were stirred on a steam bath for 24 hr. The cooled reaction mixture was extracted with hexane. The combined extracts were washed with 5% NaHCO3 and H2O and the solvent was removed under vacuum. The resulting oil, 6.1 g (92.8%), was identified as isophorone by the 2,4-dinitrophenylhydrazone derivative, melting point and mixed melting point 191°. One peak was obtained by vpc which was identical with an authentic sample of isophorone.
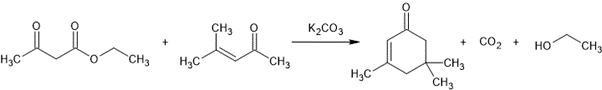
A mixture of acetoacetic ester (65 g.; 0.5 mol.), mesityl oxide (50 g.; 0.5 mol.), ethyl alcohol (60 ml), and potassium carbonate (45 g.; 0.33 mol.) is refluxed on a water bath for three hours. The ethyl alcohol is then removed by distillation, 150 ml of water is added, and reflux is continued for another hour. A double azeotrope of water and ethanol is distilled off and, after the mixture has cooled down, the organic layer is separated and distilled in vacuo. The fraction boiling at 110—115°C/10 mm is collected, giving 62 g (90% of the theoretical amount) of isophorone.
(Translated by Bitrex)
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru