2(CH3)2SO → (CH3)2S + (CH3)2SO2
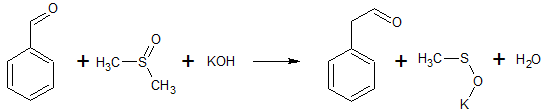
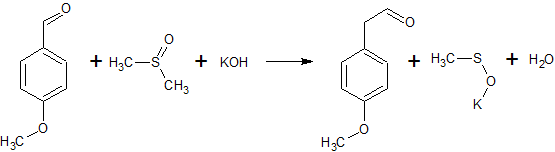






2C6H5COCl + 2CH3S(O)CH3 + CaC2 → 2C6H5COCH2S(O)CH3 + CaCl2 + C2H2
HOCH2CH2CH2CH2OH +2(CH3)2SO + 2(COCl)2 → OHCCH2CH2CHO + 2(CH3)2S + 2CO + 2CO2 + 4HCl
CH3CH2CH2CH2OH +(CH3)2SO + (COCl)2 → CH3CH2CH2CHO + (CH3)2S + CO + CO2 + 2HCl
HOC6H5 + (CH3)2SO + HCl → HOC6H4S(CH3)2Cl + H2O
K2[PtCl4] + 2(CH3)2SO → [Pt((CH3)2SO)2Cl2] + 2KCl
B2H6 + 2(CH3)2SO → (((CH3)2SO)2BH2)[BH4]
CH3COCl + CH3SOCH3 → ClCH2SCH3 + CH3COOH