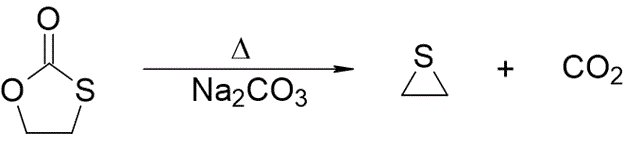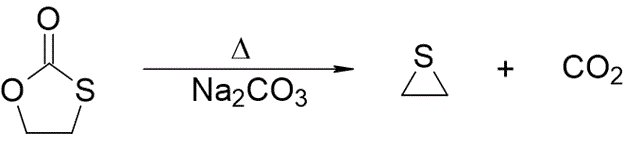Main page (Russian)
 Search in database (English)
Search in database (English)
Properties of substance:
thiirane
Group of substances:
organic
Physical appearance:
liquidEmpirical formula (Hill's system for organic substances):
C2H4SMolar/atomic mass: 60.11
Boiling point (°C):
55Decomposition temperature (°C):
250Solubility (g/100 g of solvent):
diethyl ether: difficulty soluble [Ref.]
ethanol: difficulty soluble [Ref.]
Density:
1.0368 (0°C, g/cm3)
Synthesis 1:
Reference: Reynolds, D. D. A New Method for the Preparation of Ethylene Sulfide / Journal of the American Chemical Society. - 1957. - Vol. 79, No. 18 pp. 4952 [doi: 10.1021/ja01575a033]
Thirty-six grams of monothiolethylene carbonate and 0.36 g. of anhydrous sodium carbonate were heated to 200°. The decomposition proceeded smoothly to yield ethylene sulfide, which was collected in a Dry-Ice trap (19.0 g., 85%, n(25, D) 1.4898). There was no polymerization and practically no residue remaining in the flask. Numerous other runs were made in which the yields varied from 80—88%.
Standard molar enthalpy (heat) of formation ΔfH0 (298.15 K, kJ/mol):
82 (g)Standard molar Gibbs energy of formation ΔfG0 (298.15 K, kJ/mol):
96.8 (g)Standard molar entropy S0 (298.15 K, J/(mol·K)):
255.2 (g)Molar heat capacity at constant pressure Cp (298.15 K, J/(mol·K)):
53.3 (g)LD50 (mg/kg):
35.6 (mice, oral)
References:
- CRC Handbook of Chemistry and Physics. - 90ed. - CRC Press, 2010. - pp. 5-22
- Справочник химика. - 2 изд., Т. 2. - Л.-М.: Химия, 1964. - pp. 1144-1145 [Russian]
- Успехи химии. - 2000. - Т.69, №1. - pp. 93 [Russian]
- Химия: справочное руководство. - Под ред. Гаврюченкова Ф.Г., Курочкиной М.И. и др. - Л.: Химия, 1975. - pp. 359 [Russian]
Your requests if no data into database
© Collected Ruslan Anatolievich Kiper, burewestnik@mail.ru